Pure and Pseudo-Pure fluid properties#
Introduction#
Nearly all the fluids modeling in CoolProp are based on Helmholtz energy formulations. This is a convenient construction of the equation of state because all the thermodynamic properties of interest can be obtained directly from partial derivatives of the Helmholtz energy.
It should be noted that the EOS are typically valid over the entire range of the fluid, from subcooled liquid to superheated vapor, to supercritical fluid.
Annoyingly, different authors have selected different sets of nomenclature for the Helmholtz energy. For consistency, the nomenclature of Lemmon will be used here. Also, some authors present results on a mole-basis or mass-basis, further complicating comparisons.
Thermodynamic properties of Fluid#
In general, the EOS are based on non-dimensional terms \(\delta\) and \(\tau\), where these terms are defined by
where \(\rho_c\) and \(T_c\) are the critical density of the fluid if it is a pure fluid. For pseudo-pure mixtures, the critical point is typically not used as the reducing state point, and often the maximum condensing temperature on the saturation curve is used instead.
The non-dimensional Helmholtz energy of the fluid is given by
where \(\alpha^0\) is the ideal-gas contribution to the Helmholtz energy, and \(\alpha^r\) is the residual Helmholtz energy contribution which accounts for non-ideal behavior. For a given set of \(\delta\) and \(\tau\), each of the terms \(\alpha^0\) and \(\alpha^r\) are known. The exact form of the Helmholtz energy terms is fluid dependent, but a relatively simple example is that of Nitrogen, which has the ideal-gas Helmholtz energy of
and the non-dimensional residual Helmholtz energy of
and all the terms other than \(\delta\) and \(\tau\) are fluid-dependent correlation parameters.
The other thermodynamic parameters can then be obtained through analytic derivatives of the Helmholtz energy terms. For instance, the pressure is given by
and the specific internal energy by
and the specific enthalpy by
which can also be written as
The specific entropy is given by
and the specific heats at constant volume and constant pressure respectively are given by
The EOS is set up with temperature and density as the two independent properties, but often other inputs are known, most often temperature and pressure because they can be directly measured. As a result, if the density is desired for a known temperature and pressure, it can be obtained iteratively. The following algorithm is used to obtain a reasonable guess for the initial value for the iterative solver:
If the fluid is superheated, use a guess of ideal gas (\(\rho=p/(RT)\))
If the fluid is subcooled, use a guess of saturated liquid density
If the fluid is supercritical, use a guess of ideal gas (\(\rho=p/(RT)\))
No solution for density as a function of temperature and pressure if the fluid is two-phase
The documentation of the CoolProp.CoolProp module, or the CoolProp.State module are also available.
List of Fluids#
Note
You can click on the fluid name to get more information about the fluid, or click on a bracketed reference to be taken to the reference for the fluid
Name |
EOS |
\(c_{p0}\) |
\(\lambda\) |
\(\eta\) |
melt |
\(\sigma\) |
|---|---|---|---|---|---|---|
[1] |
[2] |
|||||
[3] |
[2] |
|||||
[4] |
[5] |
[5] |
||||
[6] |
[7] |
[8] |
[2] |
|||
[9] |
[5] |
[5] |
[9] |
[2] |
||
[10] |
[11] |
[12] |
[2] |
|||
[13] |
[14] |
[15] |
[13] |
[2] |
||
[3] |
[16] |
[2] |
||||
[3] |
[2] |
|||||
[17] |
[18] |
[19] |
[2] |
|||
[20] |
[20] |
[21] |
||||
[22] |
[23] |
[24] |
[21] |
|||
[25] |
[21] |
|||||
[26] |
[21] |
|||||
[27] |
[21] |
|||||
[28] |
[2] |
|||||
[25] |
||||||
[29] |
[21] |
|||||
[30] |
[21] |
|||||
[31] |
[32] |
[2] |
||||
[33] |
[34] |
[34] |
[33] |
[2] |
||
[35] |
[36] |
[37] |
[38] |
[2] |
||
[39] |
[40] |
[21] |
||||
[41] |
[41] |
[2] |
||||
[44] |
[44] |
[44] |
[2] |
|||
[45] |
||||||
[46] |
[47] |
[47] |
[48] |
|||
[49] |
[50] |
[51] |
[52] |
[2] |
||
[53] |
[54] |
[55] |
[52] |
[2] |
||
[56] |
||||||
[3] |
[57] |
[2] |
||||
[58] |
[59] |
[60] |
[58] |
[2] |
||
[1] |
[2] |
|||||
[3] |
[2] |
|||||
[3] |
[23] |
[24] |
[61] |
[2] |
||
[3] |
[62] |
[2] |
||||
[63] |
[21] |
|||||
[26] |
[21] |
|||||
[26] |
[21] |
|||||
[63] |
[21] |
|||||
[21] |
||||||
[65] |
[66] |
[67] |
[68] |
[2] |
||
[69] |
[70] |
[71] |
[69] |
[2] |
||
[72] |
[21] |
|||||
[72] |
||||||
[72] |
[21] |
|||||
[72] |
[21] |
|||||
[72] |
[21] |
|||||
[73] |
[74] |
[2] |
||||
[3] |
[21] |
|||||
[75] |
[5] |
[5] |
[75] |
[2] |
||
[3] |
[2] |
|||||
[76] |
||||||
[28] |
||||||
[53] |
||||||
[5] |
[5] |
[79] |
[2] |
|||
[28] |
||||||
[53] |
[54] |
[55] |
[79] |
[2] |
||
[80] |
[81] |
[81] |
[61] |
[2] |
||
[20] |
[20] |
[2] |
||||
[82] |
[82] |
[83] |
[84] |
[2] |
||
[85] |
[2] |
|||||
[86] |
[86] |
[2] |
||||
[87] |
||||||
[3] |
[81] |
[81] |
[2] |
|||
[85] |
[83] |
[84] |
[2] |
|||
[88] |
[88] |
[89] |
[90] |
[2] |
||
[91] |
[92] |
[93] |
||||
[94] |
[95] |
[92] |
[2] |
|||
[96] |
[95] |
[92] |
[21] |
|||
[97] |
[93] |
|||||
[98] |
[81] |
[92] |
[2] |
|||
[97] |
||||||
[99] |
[100] |
[101] |
[2] |
|||
[86] |
[86] |
[81] |
[81] |
[2] |
||
[102] |
||||||
[103] |
[83] |
[81] |
[2] |
|||
[87] |
||||||
[86] |
[86] |
[81] |
[81] |
[2] |
||
[3] |
[81] |
[81] |
[2] |
|||
[3] |
[81] |
[81] |
[2] |
|||
[104] |
[83] |
[84] |
[21] |
|||
[105] |
[106] |
[92] |
[2] |
|||
[107] |
[2] |
|||||
[86] |
[86] |
[2] |
||||
[3] |
[81] |
[81] |
[2] |
|||
[108] |
[83] |
[92] |
[2] |
|||
[87] |
[81] |
[81] |
[2] |
|||
[109] |
[110] |
[110] |
[2] |
|||
[111] |
[81] |
[81] |
[2] |
|||
[112] |
[81] |
[81] |
[2] |
|||
[113] |
[2] |
|||||
[114] |
[81] |
[92] |
[2] |
|||
[115] |
[81] |
[92] |
[2] |
|||
[87] |
[2] |
|||||
[29] |
[21] |
|||||
[116] |
[117] |
[118] |
[119] |
|||
[116] |
[117] |
[118] |
[119] |
|||
[3] |
[2] |
|||||
[116] |
[117] |
[118] |
[119] |
|||
[116] |
[117] |
[118] |
[119] |
|||
[86] |
[86] |
[81] |
[81] |
[2] |
||
[120] |
||||||
[121] |
[2] |
|||||
[122] |
[123] |
[124] |
[2] |
|||
[3] |
[125] |
[126] |
[2] |
|||
[127] |
[128] |
[129] |
[130] |
[2] |
||
[3] |
[2] |
|||||
[1] |
[21] |
|||||
[39] |
[40] |
[131] |
[21] |
|||
[58] |
[132] |
[133] |
[58] |
[2] |
||
[3] |
[134] |
[135] |
[2] |
|||
[136] |
[137] |
[137] |
[2] |
|||
[138] |
[139] |
[140] |
[141] |
[2] |
||
[142] |
[143] |
[144] |
[2] |
|||
[3] |
[134] |
[135] |
[2] |
|||
[145] |
[134] |
[135] |
[2] |
|||
[146] |
[23] |
[67] |
[61] |
[2] |
||
[147] |
[148] |
[149] |
[61] |
[2] |
||
[150] |
[21] |
|||||
[39] |
[40] |
[151] |
[21] |
|||
[39] |
[40] |
[152] |
[21] |
|||
[1] |
[21] |
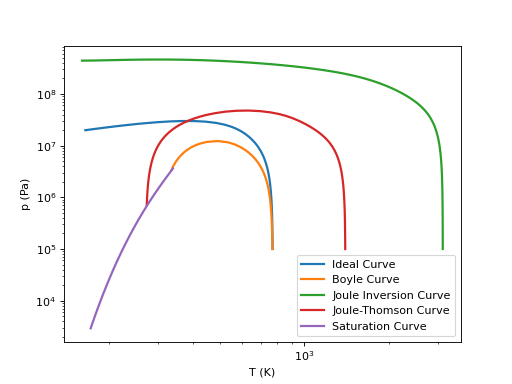
Ideal Curves#
The so-called ideal curves demonstrate the good (or not!) extrapolative behavior of the equation of state. A few common curves are defined. They should ideally look similar to the figure shown below. Lemmon [99] provides a solid coverage of the ideal curves.
Ideal Curve#
where \(Z\) can be given by
Boyle Curve#
Defined by
which can be expanded as (p can be expressed as a function of \(T\) and \(v\))
Joule Inversion Curve#
Defined by
which can be expanded as (p can be expressed as a function of \(T\) and \(v\))
Joule-Thomson Curve#
Defined by
which can be expanded as (v can be expressed as a function of \(T\) and \(p\))
where
Ideal Curves for Refrigerant R125#
(Source code, png, .pdf)